- Explain Functional group giving examples.
- An atom or a group of atoms which determines the characteristic reactions of organic compounds is called the functional group.
- Different organic compounds having same functional group show similar chemical properties.
- Some common functional groups are:
- Write a brief note on Alcohol group.
- Organic compounds containing hydroxyl (-OH) functional group are called alcohols.
- If one hydrogen atom of an alkane is displaced by the hydroxyl group, then corresponding alcohol compound is obtained.
- The general formula of alcohol group is CnH2n+1OH (written as R-OH, where R = alkyl group).
- Suffix -ol is used after removing last alphabet 'e' from the name of the hydrocarbon to give name to the corresponding alcohol.
- Thus, methanol corresponds to methane and ethanol corresponds to ethane.
- Examples: Methanol (CH3OH), ethanol (CH3CH2OH or C2H5OH), propanol (C3H7OH), butanol (C4H9OH), etc.
- Explain fermentation reaction and its importance (Write a note on fermentation).
- The reaction of formation of simple substances by slow decomposition of organic compounds through enzymes in absence of oxygen is called fermentation.
- Formation of curd from milk, the reaction occurring in dough of idli, formation of ethanol from the juice of fruits are examples of fermentation process.
- Fermentation is thus important in everyday life for the preparation of food items like idli, dhokla, handva, etc. as well as curd.
- Industrially, many important chemicals like ethanol and acetic acid are obtained by fermentation.
- For example, fermentation of sugar by enzymes invertase and zymase produces ethanol in two stages.
- During first stage, sugar (from molasses or fruit juice) is converted to simple sugars glucose and fructose by enzyme invertase.(reaction to be posted)
- During second stage, glucose (or fructose) is converted to ethanol by enzyme zymase and carbon dioxide gas is released.(reaction to be posted)
- Describe preparation of ethanol by fermentation.
- Describe modern method of industrial production of ethanol.
- Write properties and uses of ethanol.
- PHYSICAL PROPERTIES
- Pure ethanol is colourless liquid.
- It is highly soluble in water.
- Its boiling point is 351 K.
- CHEMICAL PROPERTIES
- Combustibility
Ethanol is highly combustible and burns rapidly with a blue flame forming carbon dioxide and water. - Reaction with sodium metal
When ethanol reacts with sodium metal, sodium ethoxide and dihydrogen gas are produced. - Reaction with organic acid
Ethanol reacts with ethanoic acid (acetic acid) in presence of concentrated sulphuric acid and produces ethyl acetate (an ester) which has sweet fruity smell. - Oxidation
Alkaline KMNO4 oxidizes ethanol to acetic acid.
- Combustibility
- USES
- As a solvent in industry.
- Ethanol is used in lacquers, varnish and perfumes.
- In medicine as antiseptic for dressing of boils and wounds.
- As a fuel in spirit lamp in laboratory.
- Ethanol containing 5% water is known as rectified spirit and is used for making outer surface of the body germ-free.
- Describe ethanol as toxic substance or describe harmful effects of ethanol.
- Ethanol is a toxic alcohol.
- Those who consume ethanol lose sensitivity and balance of body.
- It affects liver and leads to a disease called Cirrhosis of liver which may cause death.
- Ethanol is the main constituent of alcoholic drinks.
- If it is consumed in small amount it works as a stimulant.
- Ethanol is absorbed through mucosa of stomach and mixes with the blood. If concentration of alcohol in blood increases beyond 0.3%, it is harmful.
- 90% of alcohol absorbed in cells is converted to acetaldehyde by oxidation. Acetaldehyde is then oxidized to acetic acid and then finally, carbon dioxide and water are formed.
- The toxic effect of alcohol is due to acetaldehyde which gives the feeling of vomiting and the person loses balance and becomes unconscious.
- In the liver of alcohol drinker, the level of enzyme P-450 becomes high and it gives temptation to drink more alcohol. Thus, it creates addiction.
- A medicine called disulfiram is given to alcohol-addicts. This medicine prevents further oxidation of acetaldehyde. Acetaldehyde gives vomiting sensation and nausea and the addict slowly develops hatred towards alcohol.
- To prevent consumption of alcohol, toxic adulterants like methanol are added to it. If ethanol containing methanol (Lattha) is consumed, person loses eye-sight.
- Write a brief note on carbonyl group.
- >C=O is called carbonyl group.
- Aldehydes and ketones are organic compounds containing carbonyl group.
- In aldehydes, the carbon of carbonyl group is attached with one alkyl (-R) group and one hydrogen atom except in formaldehyde (methanal) where the carbon atom of carbonyl group is attached to two hydrogen atoms.
- In ketones, the carbon atom of carbonyl group is attached to two alkyl groups (R and R', where R and R' are similar or different alkyl groups).
- For nomenclature of aldehyde compounds, the last alphabet 'e' from the longest hydrocarbon chain is replaced by the suffix 'al'. Thus, an aldehyde corresponding to methane is called methanal and that corresponding to ethane is called ethanal.
- For nomenclature of ketone compounds, the last alphabet 'e' from the longest hydrocarbon chain is replaced by the suffix 'one'. Thus ketone corresponding to propane is called propanone.
- A ketone containing less than three carbon atoms does not exist.
- The first four aldehyde compounds are HCHO (formaldehyde or methanal), CH3CHO (acetaldehyde or ethanal), CH3CH2CHO (propionaldehyde or propanal) and CH3CH2CH2CHO (butanaldehyde or butanal).
- The first two ketone compounds are CH3COCH3 (acetone or propanone) and CH3CH2COCH3 (methyl ethyl ketone or butanone).
- Describe preparation, properties and uses of formaldehyde (methanal).
- PREPARATION: Methanal is prepared by oxidation of methanol in presesnce of a catalyst like oxide of silver or iron.
- PHYSICAL PROPERTIES
- Methanal is a colourless, poisonous gas.
- Its boiling point is 253 K.
- It is soluble in water.
- CHEMICAL PROPERTIES
- Oxidation: Methanal is oxidized to methanoic acid by oxidizing agents like Tollen's reagent (ammoniacal silver nitrate), or Fehling's reagent.
- Reduction: Reduction of methanal by dihydrogen gas in presence of catalyst palladium(Pd) gives methanol.
- Addition Reaction With Hydrogen Cyanide (HCN): Methanal forms methanal cyanohydrin with hydrogen cyanide by addition reaction.
- Oxidation: Methanal is oxidized to methanoic acid by oxidizing agents like Tollen's reagent (ammoniacal silver nitrate), or Fehling's reagent.
- USES
- Aqueous solution of methanal (formalin) is used to preserve residues of dead animals.
- As a raw material (monomer) in preparation of plastics like bakelite and melamine.
- As a raw material in preparation of urea-formaldehyde resin.
- In preparation of dyes and polymers like phenol-formaldehyde.
- Describe preparation, properties and uses of propanone (acetone).
- PREPARATION: Propanone (acetone) is prepared by the reaction of ethene with water gas in presence of the catalyst cobalt oxide at 150 bar pressure and 453 K temperature. The process is called Fischer-Tropsch process.
- PHYSICAL PROPERTIES
- Propanone is a colourless liquid possessing fragrance.
- Its boiling point is 329 K.
- It is soluble in water.
- CHEMICAL PROPERTIES
- Reduction: Reducing agents like sodium boron hydride (NaBH4) or lithium aluminium hydride (LiAlH4) reduce propanone to propan-2-ol (2-propanol).
- Oxidation: Propanone is oxidized by alkaline potassium permanganate to ethanoic acid (acetic acid).
- Addition Reaction With HCN: Propanone forms propanone cyanohydrin with hydrogen cyanide by addition reaction.
- Reduction: Reducing agents like sodium boron hydride (NaBH4) or lithium aluminium hydride (LiAlH4) reduce propanone to propan-2-ol (2-propanol).
- USES
- As a solvent in laboratory and in paint industry.
- For preparation of artificial leather and synthetic fibres.
- As nail-polish remover.
- Write a brief note on carboxylic acid group.
- Organic compounds containing -COOH functional group are called carboxylic acid compounds.
- In the nomenclature of carboxylic acids, the last alphabet 'e' from the name of the longest hydrocarbon chain is replaced by suffix '-oic acid'.
- Methanoic acid (formic acid) and ethanoic acid (acetic acid) are carboxylic acid compounds corresponding to methane and ethane, respectively.
- HCOOH, CH3COOH, CH3CH2COOH are first three compounds of this series.
- The general formula of carboxylic acids is CnH2n + 1COOH, where n = 0, 1, 2,...
- Describe preparation, properties and uses of ethanoic acid (acetic acid).
- PREPARATION
- from Ethanol: Oxidation of ethanol by fermentation in presence of enzyme acetobactor gives ethanoic acid (vinegar). The proportion of ethanoic acid obtained by this method is very less.
- From Methanol: In this modern industrial process, the reaction between methanol and carbon monoxide in presence of catalyst iodine-rhodium (I2-Rh) yields ethanoic acid.
- from Ethanol: Oxidation of ethanol by fermentation in presence of enzyme acetobactor gives ethanoic acid (vinegar). The proportion of ethanoic acid obtained by this method is very less.
- PHYSICAL PROPERTIES
- Ethanoic acid is a colourless liquid.
- It has sour smell.,li>It is soluble in water.
- Its boiling point is 391 K.
- CHEMICAL PROPERTIES
- Reaction With Metals: When ethanoic acid reacts with metals like sodium and magnesium, metal carbonate salt and dihydrogen gas are produced.
- Reaction With Base: When ethanoic acid reacts with a base like sodium hydroxide, a salt (sodium acetate) and water are produced.
- Reaction With alcohol: Ethanoic acid reacts with alcohol (ethanol) in presence of concentrated sulphuric acid and produces an ester (ethyl ethanoate) by esterification reaction.
- Reaction With Metals: When ethanoic acid reacts with metals like sodium and magnesium, metal carbonate salt and dihydrogen gas are produced.
- USES
- Vinegar prepared from acetic acid is used as a preservative in foods and to have sour taste.
- In preparation of white lead.
- As a reagent and solvent in laboratory.
- Describe the classification of polymers.
- NATURAL POLYMERS: These are polymers occurring naturally. For example ; starch, protein, nucleic acid, cotton, rubber, etc.
- SEMI-SYNTHETIC POLYMERS: These are polymers obtained from naturally occurring polymers by some chemical reaction. For example: vulcanized rubber obtained by the reaction of natural rubber with sulphur.
- SYNTHETIC POLYMERS: These are man-made polymers obtained by the polymerization of simple substances. All plastics, many fibres and synthetic rubber are synthetic polymers and are widely used in industry, electrical appliances and as an alternative to wood and metal.
- Homopolymers: When one type of innumerable simple organic monomers combine with one another through chemical bond formation to give polymeric substances, the polymer is called a homopolymer. For example, polythene formed from ethene.
- Co-polymers: When two or more types of innumerable simple organic monomers combine with one another through chemical bond formation to give polymeric substances, the polymers are called co-polymers. For example, styrene-butadiene rubber (SBR) prepared from monomers styrene and butadiene.
- Addition Polymers: These are polymers formed by combination of simple organic monomers having double bond by chemical bond formation resulting in long chain. For example, polystyrene obtained from styrene.
- Condensation Polymers: These are polymers formed by two different types of monomers which combine with one another by condensation polymerization after removal of molecules like water, ammonia, alcohol, etc. For example, Nylon 6,6 (polyamide) is a condensation polymer obtained from hexamethyl diamine and adipic acid.
- Write a note on addition polymers.
- Addition polymers are polymeric substances formed by combination of simple organic monomers having double bond by addition reaction. They have long chains of innumerable monomers of one type.
- Following are some examples of addition polymers:
- Polythene, [-CH2-CH2-]n formed from monomer ethene, CH2=CH2.
Polythene is used in toys, packaging bags, etc. - Polyvinyl chloride (PVC), [-CH2-CHCl-]n formed from vinyl chloride, CH2=CH-Cl.
Polyvinyl chloride is used in preparation of flooring tiles, rain-coats, handbags, etc. - TEFLON (polytetrafluoroethene), [-CF2-CF2-]n formed from tetrafluoroethene, CF2=CF2.
TEFLON is used in preparation of non-stick cook-wares and as an insulator. - Natural rubber is a polymer of 2-methylbuta-1,3-diene (isoprene).
Natural rubber is used in preparation of water-proof clothes, tyres of vehicles, etc. - Polybutadiene, [-CH2-CH=CH-CH2-]n, is formed from butadiene, CH2=CH-CH=CH2 and is used as an alternative of natural rubber.
- Neoprene (artificial rubber) is prepared from 2-chlorobuta-1,3-diene and is used as an insulator, in conveyer belts and in rollers for printing.
- Polythene, [-CH2-CH2-]n formed from monomer ethene, CH2=CH2.
- Write a note on rubber.
- Rubber is naturally available from rubber trees.
- Natural rubber is extracted from a colloidal suspension called rubber latex by physical and chemical reactions.
- Rubber latex is obtained by making a cut in the bark of rubber plant.
- The elastic property of natural rubber is maintained between temperatures 283 K and 333 K.
- Rubber becomes brittle below 283 K and soft above 333 K.
- It has high water- absorption capacity.
- It is resistant to non-polar solvents.
- It gets oxidized easily by oxidizing agents.
- VULCANIZED RUBBER:
- In 1983, Charles Goodyear found out that if rubber is heated with sulphur at temperatures in the range of 373 to 413 K, its properties can be modified as required.
- The process is called vulcanization and the rubber thus obtained is called vulcanized rubber.
- This reaction is slow, so zinc oxide is added to increase the rate of reaction.
- By adding 5% sulphur, vulcanized rubber suitable for tyres can be prepared.
- By adding 30% sulphur, vulcanized rubber suitable for the cases of battery can be prepared.
- Vulcanized rubber has very good elasticity, very low water-absorption property and excellent resistance to organic solvents and oxidation reaction.
- Write a brief note on condensation polymers.
- Polymers formed by condensation polymerization of two different types of simple organic monomers are called condensation polymers. The reaction involves removal of molecules like water, ammonia or alcohol.
- Polyester and polyamide (Nylon) are examples of condensation polymers.
- Nylon 6,6 is obtained by condensation polymerization of hexamethylene diamine and adipic acid.
- Write a note on polyester.
- Polyester is formed by combination of compounds containing two hydroxyl groups and two carboxylic groups.
- A polyester molecule consists of a long chain of ester group.
- Polyester fibres are mixed with cotton fibres and used in textile industry.
- Write a note on polyamide (Nylon).
- Polyamide is formed by combination of compounds containing two amine groups and two carboxylic groups.
- A polyamide molecule consists of a long chain of amide group.
- Polyamides are also known as nylons.
- Polyamide (nylon) is a thermoplastic polymer and its fibres are strong, elastic and water-resistant.
- It is used in preparation of fishing nets, ropes and in tyres.
- Write a note on bio-polymers.
- Polymers like polysaccharides, proteins, nucleic acid occurring in nature are essential for human beings. These are called bio-polymers or biopolymeric substances.
- Most of the polymeric substances used in day-to-day life (like polythene, PVC) are inert towards environmental processes. Their decomposition by natural factors is not possible so we cannot produce substances useful biologically.
- In fact, such non-biodegradable substances produce wastes which are harmful and create problems of disposal.
- In biological systems, the degradation of biopolymers is carried out mostly by hydrolysis through enzymes and to some extent by oxidation.
- Recently, the development of biodegradable polymers is being carried out with a view to obtaining suitable substances for human life system which do not create waste disposal problems after their use.
- USES: (i) Biopolymers or biodegradable polymers are used in special type of packing, orthopaedic appliances and in capsules to fill controlled drugs like PHBV (Polyhydroxy butyrate Co-β hydroxy valerate).
(ii) For taking stitches after surgical operation, dextran is used. It is a biodegradable polymeric substance. - Write a note on soap and synthetic detergents.
- A chemical substance used to remove the dirt stuck to a surface without harming the surface is called detergent.
- SOAP
- Soap is a sodium or potassium salt of fatty acid (stearic acid, oleic acid, palmitic acid, etc. are fatty acids). It contains -COONa as the functional group.
- Sodium salt is used in washing soaps and potassium salt is used in bathing soaps.
- Fatty acids are present in animal fat (mutton tallow) and vegetable oils and they give glycerol and ester compounds.
- PREPARATION OF SOAP
- When vegetable oil or animal fat is heated with sodium hydroxide, sodium salt of fatty acid (soap) and glycerol are formed. This process is called saponification.
- Method of Preparation: Take 20 ml vegetable oil in a beaker and add 20% aqueous solution of NaOH. Heat the mixture for some time and stir it. When the mixture becomes viscous, add 5 to 10 gm of common salt (NaCl). On cooling, the insoluble part will float in the upper part of the mixture. Take the insoluble mass in another container and allow it to become dry. add antiseptic drug, fragrant substances and filler as needed. Prepare cakes of soap.
- When vegetable oil or animal fat is heated with sodium hydroxide, sodium salt of fatty acid (soap) and glycerol are formed. This process is called saponification.
- DETERGENT
- Detergents are sodium salts of organic sulphonic acids. They contain -SO3Na (sodium sulphonate) as the functional group.
- Since detergents do not form precipitates with Ca+2 and Mg+2 ions present in hard water, their cleansing effect is better than that of the soap.
- Their consumption is less compared to soap, therefore, their use has increased.
- CLEANSING PROCESS OF SOAP AND DETERGENT
- In soap, -COONa functional group is attached to hydrocarbon. In detergent, -SO3Na functional group is attached to hydrocarbon.
- The cleansing process of soap and detergent is the same.
- There are two parts in the structure of soap and detergent.
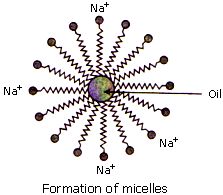
- THe long hydrocarbon chain is known as nonpolar tail because it does not possess attraction towards water but possesses attraction towards dirt or stain.
- The other negatively charged part (-COONa in soap and -SO3Na in detergent) is known as polar head. It possesses attraction towards water.
- When the solution of soap or detergent is applied on the surface having dirt or oily stains, the nonpolar part is attracted by oily stain or dirt.
- The polar part remains in water due to its attraction towards water.
- Due to these opposing forces, dirt or stain is removed from the surface and water becomes dirty.
- The spherical structure formed around the stain is called 'micelle'.
- In soap, -COONa functional group is attached to hydrocarbon. In detergent, -SO3Na functional group is attached to hydrocarbon.
ANS
ANS
ANS
ANSRefer to Q. 3 for answer.
ANS
Modern method of industrial production of ethanol involves hydration of ethene in presence of concentrated sulphuric acid.
ANS
ANS
ANS
ANS
ANS
ANS
ANS
ANS
ANS
ANS
ANS
ANS
ANS
ANS
ANS
ANS



























Hiv disease for the last 3 years and had pain hard to eat and cough are nightmares,especially the first year At this stage, the immune system is severely weakened, and the risk of contracting opportunistic infections is much greater. However, not everyone with HIV will go on to develop AIDS. The earlier you receive treatment, the better your outcome will be.I started taking ARV to avoid early death but I had faith in God that i would be healed someday.As a Hiv patent we are advise to be taking antiretroviral treatments to reduce our chance of transmitting the virus to others , few weeks ago i came on search on the internet if i could get any information on Hiv treatment with herbal medicine, on my search i saw a testimony of someone who has been healed from Hiv her name was Achima Abelard and other Herpes Virus patent Tasha Moore also giving testimony about this same man,Called Dr Itua Herbal Center.I was moved by the testimony and i contacted him by his Email.drituaherbalcenter@gmail.com We chatted and he send me a bottle of herbal medicine I drank it as he instructed me to.After drinking it he ask me to go for a test that how i ended my suffering life of Hiv patent,I'm cured and free of Arv Pills.I'm forever grateful to him Drituaherbalcenter.Here his contact Number +2348149277967...He assure me he can cure the following disease..Hiv,Cancer,Herpes Virus,Epilepsy, fibromyalgia ,ALS,Hepatitis,Copd,Parkinson disease.Genetic disease,Fibrodysplasia Ossificans Progressiva,Factor V Leiden Mutation ,Fatal Familial Insomnia ,Dupuytren's disease,Fibrodysplasia Ossificans Progressiva,Fluoroquinolone Toxicity Syndrome,Inflammatory bowel disease ,Huntington's disease ,Diabetes,Fibroid...
ReplyDeleteHOW I GOT CURED OF HERPES VIRUS.
ReplyDeleteHello everyone out there, I am here to give my testimony about a herbalist called Dr Imoloa. I was infected with herpes simplex virus 2 in 2013, I went to many hospitals for cure but there was no solution, so I was thinking on how I can get a solution out so that my body can be okay. One day I was in the pool side browsing and thinking of where I can get a solution. I go through many websites where I saw so many testimonies about dr imoloa on how he cured them. I did not believe but I decided to give him a try, I contacted him and he prepared the herpes for me which I received through DHL courier service. I took it for two weeks after then he instructed me to go for check up, after the test I was confirmed herpes negative. Am so free and happy. So, if you have problem or you are infected with any disease kindly contact him on email drimolaherbalmademedicine@gmail.com. Or / whatssapp --+2347081986098.
This testimony serve as an expression of my gratitude. He also has
herbal cure for, FEVER, BODY PAIN, DIARRHOEA, MOUTH ULCER, MOUTH CANCER FATIGUE, MUSCLE ACHES, LUPUS, SKIN CANCER, PENILE CANCER, BREAST CANCER, PANCREATIC CANCER, CHRONIC KIDNEY DISEASE, VAGINAL CANCER, CERVICAL CANCER, DISEASE, JOINT PAIN, POLIO DISEASE, PARKINSON'S DISEASE, ALZHEIMER'S DISEASE, BULIMIA DISEASE, INFLAMMATORY JOINT DISEASE CYSTIC FIBROSIS, SCHIZOPHRENIA, CORNEAL ULCER, EPILEPSY, FETAL ALCOHOL SPECTRUM, LICHEN PLANUS, COLD SORE, SHINGLES, CANCER, HEPATITIS A, B. DIABETES 1/2, HIV/AIDS, CHRONIC RESPIRATORY DISEASE, CARDIOVASCULAR DISEASE, NEOPLASMS, MENTAL AND BEHAVIOURAL DISORDER, CHLAMYDIA, ZIKA VIRUS, EMPHYSEMA, TUBERCULOSIS LOW SPERM COUNT, ENZYMA, DRY COUGH, ARTHRITIS, LEUKAEMIA, LYME DISEASE, ASTHMA, IMPOTENCE, BARENESS/INFERTILITY, WEAK ERECTION, PENIS ENLARGEMENT. AND SO ON.
website- www.drimolaherbalmademedicine.wordpress.com
Get science homework help at TutorEye. Post your science homework questions and get answers from qualified tutors.
ReplyDelete